EU Novel Foods & CBD In 2024
Since its inception, the UK CBD oil industry has been left to its own devices to self regulate and ensure CBD products are fit for sale.
However, since early last year, it’s been clear that change was around the corner with the official recognition of CBD as a novel food at an EU level.
This categorisation means CBD products now (technically) require pre-authorisation before going on sale and that all current ingestible CBD on the market in the EU needs to be approved.
Still, enforcement is up to each EU member state. To date, the levels of implementation have been less than uniform, with some countries being much more stringent in the process than others.
While the UK has left the European Union it will still comply with the legislation. The Food Standards Agency (FSA), has announced that all current ingestible CBD on the UK market (excluding Scotland) will need to be submitted for addition to the novel foods catalog by March 31st, 2021. After this date, CBD oil is legal in the UK only if it has an accompanying novel food application submitted.
Any consumable CBD products which are not submitted for addition to the novel food catalogue by this date will not be allowed to remain on sale in the UK market…We will dive into what this means for the industry shortly.
But first, what is novel food regulation, and why does it apply to CBD?
What makes a food novel?
Novel foods are EU foods which have not been widely consumed before May 15th 1997 in the EU.
The primary reason a food is classed as novel is that there is a lack of historical use in regard to its consumption and as such little empirical data if that ingredient is stable when used in food products.
The EU novel foods regulation has three primary stipulations, where the food must not:
- Present a danger to the consumer.
- Mislead the consumer.
- Be nutritiously disadvantageous for the consumer if they are intended to replace another ingredient.
What are examples of novel foods?
The regulation is a European Commission mandate which applies to countries within the European Union. Although, the UK will enforce the directive after the Brexit transition period.
Novel foods examples include:
- New food ingredients such as plant sterol esters used in butter substitutes.
- Foods produced using a new process without documented historical use.
- Foods consumed elsewhere but not commonly in the EU (before May 1997) such as chia seeds and baobab.
What’s needed to become an approved food?
Any product containing a novel food ingredient will need to pass a rigorous safety assessment before being placed on sale.
The work required for the approval of a new novel food is lengthy and costly where the process involves the creation of a dossier of scientific data points, which can take a year to collate.
Add in the cost of legal advice to ensure the application contains what’s required for approval, and the entire process can end up costing in excess of a hundred thousand pounds.
It’s also widely expected that any new application will take 12-18 months before approval and might require revisions prior to being rubber-stamped by the European Commission.
At the date of writing, there is not a single CBD novel food application which has been approved.
New CBD regulation in the EU
With the classification of CBD as a novel food in January 2019, the impending change has been inevitable.
Countries such as Germany have started to exercise more immediate action for new food regulation, whereas others such as the UK have offered a window of grace to submit a novel foods application. This is likely associated with the size of the UK CBD market, with the UK being the largest market in the EU by far.
In the UK this undercurrent of change is technically owned by the Food Standards Authority. Still, it appears to have been spurred on by the creation of the Association of Cannabinoid Industry in late 2019, an affiliated company of the Centre of Medicinal Cannabis.
While it’s true that in the UK any CBD product which is considered an ingestible will require approval, raw CBD material manufactures will be held to the highest levels of compliance.
What does this mean? Depending on where in the chain the business is, different levels of regulatory oversight will apply. For example:
Raw material manufacturers must carry out the most extensive amount of due diligence and work to comply with the Novel Foods regulation. They must gain appropriate levels of safety and stability data. Combined with harmonised CBD testing methods to submit a novel foods application. This tranche of the CBD industry will need to complete this work and submit it before 31st March 2021 to keep their products on the market after that.
Manufactures of finished products must liaise with their raw material suppliers to ensure they are engaging in a novel foods application and that they have the required safety and stability data.
White label brand owners must consult with their suppliers to ensure they are only using raw materials which are being submitted as part of the Novel Foods process. Final products will need to show benchmarked levels of stability for the end user in order to be approved for use.
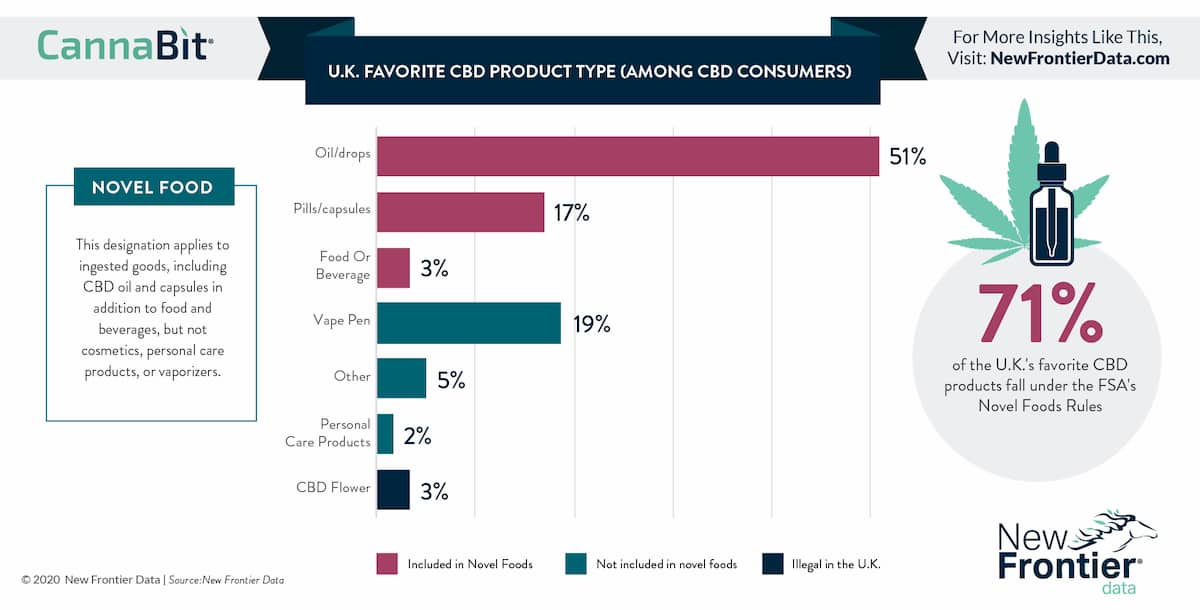
Who will enforce novel foods in the UK?
While the FSA is in charge of the overarching UK regulation when it comes to anything related to foods, enforcement is at the local level.
As a result, it will be up to local trading standards and environmental health officers to engage with brands and retailers in regards to novel foods enforcement.
Summary
- Novel foods regulation is a European Commission led mandate which applies to any new food which isn’t consumed in mass in the European Union prior to early 1997.
- Foods considered novel require pre-authorisation before they are allowed to be sold. CBD is a unique example as it’s been on sale prior to being considered a novel food.
- The authorisation process isn’t straight forward and it is expensive.
- The FSA has outlined a grace period of until March 31st 2021 to submit a novel foods application. Any ingestible CBD sold after this period will need to be backed by a novel food application for sale in England, Wales and Northern Ireland.
- CBD products which are not considered foods such as vape or cosmetic products do not require novel food approval to be sold in the European Union.
- In the UK enforcement is at the local county level, primarily by trading standards. How this will work is currently an unknown.

